HVAC Science for Homeowners
Want to Know How It All Works?
Once you understand the science behind HVAC, you can better understand how your home is heated and cooled.
Conservation of Energy
Energy Balancing
Why Hot Air Rises?
Imperfect Systems

Conservation of Energy
If you liked science class, then you might remember Newton’s Third Law of Motion: For every action, there is an equal and opposite reaction. A simple example of this law of motion is paddling in a canoe. While the paddle pushes water in one direction, the canoe moves in the opposite direction.
There is a similar law of equality in the science that fuels HVAC: thermodynamics. The first law of thermodynamics states that heat is a form of energy, and energy can neither be created nor destroyed. Let’s break that down.
Energy always has to come from somewhere. When you boil water on the stove, it doesn’t happen magically. To simplify, energy is transferred from the stove to the pot, and then from the pot to the water. Energy is just transferring around from one set of atoms to another.
Energy Balancing
Now, let’s take it a step further. The second law of thermodynamics states that energy will always strive for equilibrium. In other words, energy is always trying to even itself out amongst everything in its direct surroundings. Think of dropping water onto a sponge: the water will continuously seep into a sponge and the water will eventually evenly dampen the area.
So, how does this work for air? Remember that everything on Earth: your furniture, your cell phone, and even your own human body–it’s all made up of atomic molecules. Air, too, is made of up of atoms. With that in mind, let’s go back to our boiling water example.
The boiling water in our pot will generate steam. Steam on its own is hot–but it doesn’t stay in a cloud of steam forever. It seeks equilibrium–and although you can’t see it with your eyes, the steam is constantly transferring energy with the cooler air around it. So your kitchen area will get warmer.

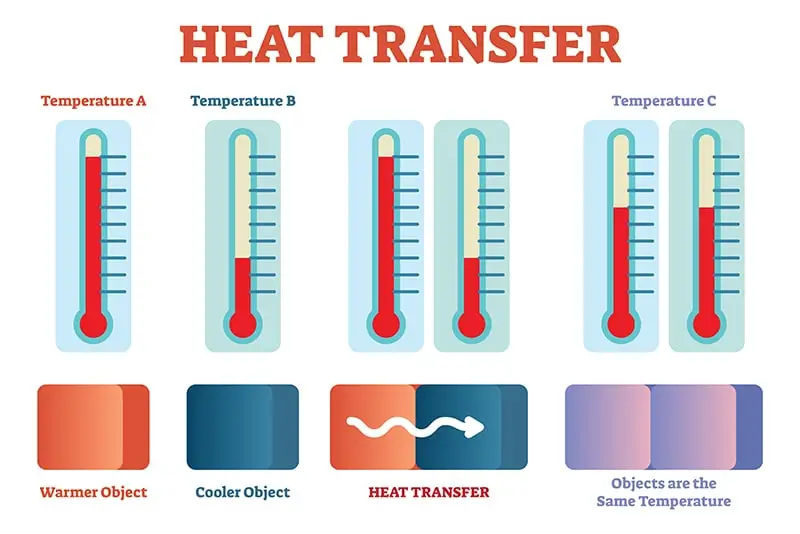
Energy Balancing in Reverse
A reverse example of this is taking the boiling water off of the stove.
When the water gets cool, it’s not losing heat–it’s just transferring it! Energy is still being transferred, but instead of from the hot stove to the pot, the energy transfer is happening from the stove to the surrounding air.
Energy is transferred until there is equilibrium: the pot and water inside are room temperature because the heat they once contained has been transferred to the surrounding air.
Now you have plenty of knowledge about thermodynamics. You can now smartly explain lots of phenomena. At your next meeting you can tell your coworker, “Your coffee isn’t getting cold, it’s just transferring energy!”
Why Does Hot Air Rise?
Cold air and warm air have the same number of molecules. As cold air gets warmer, the individual molecules that make up the air start moving away from each other. This makes the air less dense.
Molecules will naturally move through a space, with cold, denser air molecules near the floor and the hot, less dense air molecules will rise to the ceiling.
By the way, this is why steam rises away from a cup of tea. The steam is less dense than the relatively cooler air around it.


Imperfect Systems
If you’re following along so far you may be wondering, if energy is constantly trying to make itself even, why is it cool and comfortable in one space and hot in another? In fact, if the laws above are true, shouldn’t the whole world and everything in it be the exact same temperature?
These scientific laws are based on perfectly isolated or “closed” systems. A perfect, closed system does not truly exist. An example of a nearly-closed system would be a pot of boiling water with a lid on it. The water inside is a closed system. However, even a lid seeps some of the steam a little. And, the water inside is still reacting with the pot. The pot is still reacting with the surrounding air.
It’s also true in your house. A closed door to a bedroom creates a more closed system–keeping the energy transfer mostly happening within the space. If all doors and windows in your house are open… Well, that’s a very open system, because now the outdoors is part of the system!
The good news is, professionals in HVAC science are trained to understand–and anticipate–how systems change.
